To close IATF non-conformities received from the IATF auditor, upload the JSON file in the NC Cara portal. CARA in IATF is the Common Audit Report Application. This is the application on the IATF Global oversight website. There are 7 steps for closing the IATF non-conformity on the NC CARA portal.
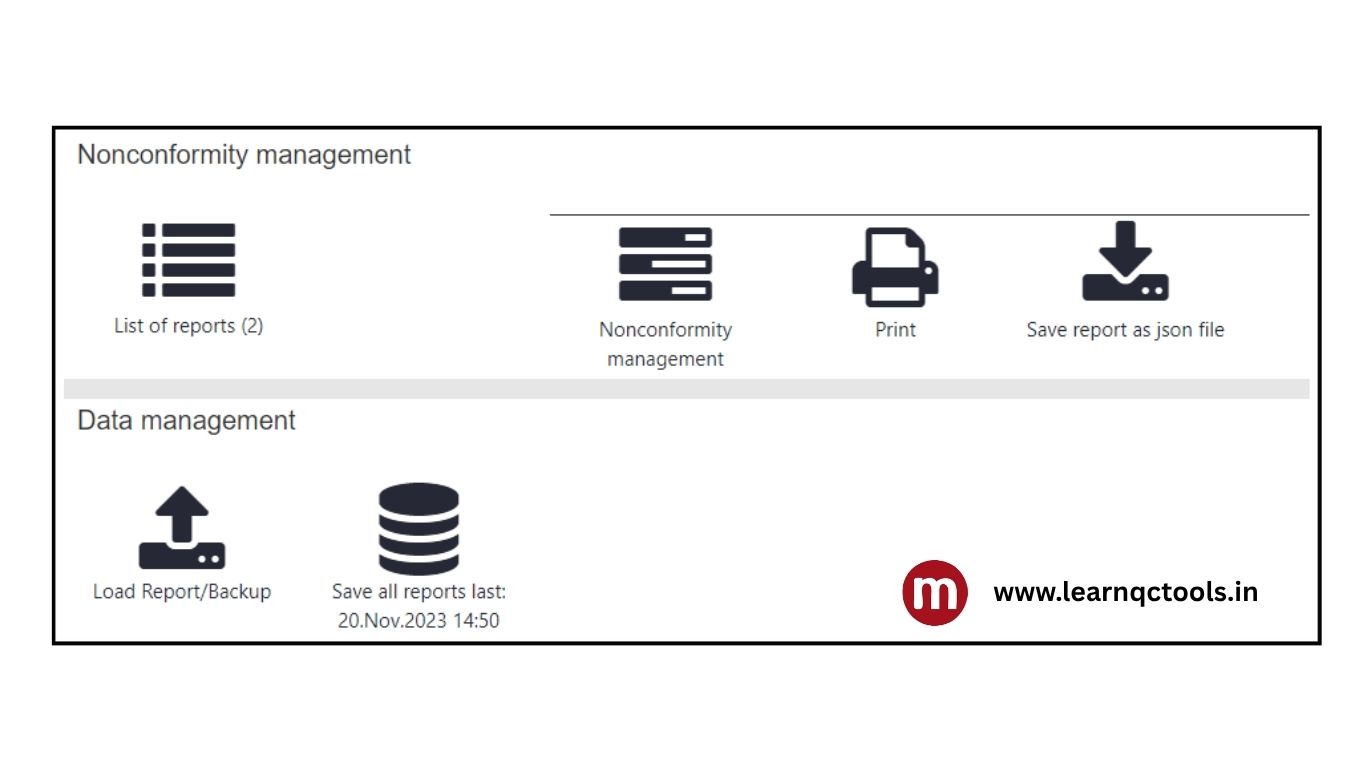
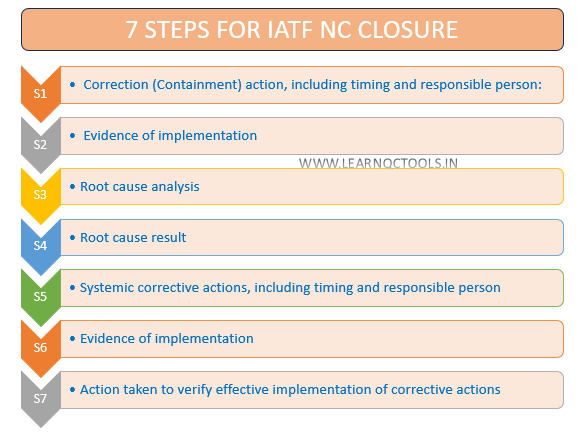
7 steps to close the IATF audit Non-Conformity on the NC CARA Portal:
S1: Correction (Containment) action, including timing and responsible person
In this step, define the containment actions to close the non-conformity given by the auditor. Also define the responsible person and the Target date.
S2: Evidence of implementation
In this step, describe the containment action taken as described in Step 1 to close the non-conformity.
S3: Root Cause Analysis
In this section, we do a why why analysis to find out the root cause of the problem. Also attach the Root cause analysis pdf file.
S4: Root cause result
In this step, write about the final root cause of the non-conformity. The final root cause should be systematic. Also attach the root cause result pdf file. The root cause should not be operator training, tool breakdown etc.
S5: Systemic corrective actions, including timing and responsible person
In this step, define the systematic corrective actions so that the non-conformity will not repeat. Systematic corrective action is defined in documents such as Quality procedures, work instructions etc. Also define the responsibility and target date for the corrective actions.
S6: Evidence of implementation
In this step, describe the systematic corrective actions, which are defined in Step 5. Also attach the pdf file of the systematic corrective action.
S7: Action taken to verify the effective implementation of corrective actions
In this step, write about the actions to verify the effective implementation of corrective actions taken. Also attach the pdf file of verification. Generally, an internal audit is done to verify the corrective action for the non-conformity. Lastly, do MRM also to inform the top management about the closure of all non-conformities.
IATF NC Closure Examples:
Statement of nonconformity
The Process of Purchase was not found to be fully effective.
Requirement as per IATF 16949:
Supplier Monitoring (8.4.2.4): The organization shall have a documented process and criteria to evaluate supplier performance to ensure conformity of externally provided products, processes, and services to internal and external customer requirements. At a minimum, the following supplier performance indicators shall be monitored:
- delivered product conformity to requirements;
- customer disruptions at the receiving plant, including yard holds and stop ships;
- delivery schedule performance;
- special status customer notifications related to quality or delivery issues;
- dealer returns, warranty, field actions, and recalls.
Objective evidence
During the audit, observed supplier performance was not found to be monitored for the RM supplier…………..
Justification for classification
Isolated gap; the concerned person was found aware. 5 other samples were checked and found ok, with no impact on delivery, hence minor
| Step No | Step Description | Closure Evidence |
|---|---|---|
| S1 | Correction (Containment) action, including timing and responsible person | Raw material supplier performance monitoring sheet to be updated and sent to the supplier by Mr……….. on Date……….. |
| S2 | Evidence of implementation | Raw material Supplier Performance Rating Sheet prepared. |
| Evidence of implementation file | RM Supplier Performance Rating pdf | |
| S3 | Root Cause Analysis | Why-01: RM Supplier performance monitoring not done Why-02: Customer approved RM supplier monitoring not done Why-03: In the procedure about Raw Material Supplier performance monitoring is not defined. Why-04: Supplier development procedure not effective. |
| Does the root cause impact other similar processes or products? | Yes | |
| Please describe how the root cause impacts other processes. | Other parts quality received from other suppliers will not be good | |
| Root cause analysis files | Root cause analysis files pdf | |
| S4 | Root cause result | Supplier development procedure is not effective |
| Root cause result files | Root cause analysis result files pdf | |
| S5 | Systemic corrective actions, including timing and responsible person | Supplier development procedure to be updated by Mr……….. on Date ………… |
| Corrective action files | Supplier development procedure pdf | |
| S6 | Evidence of implementation | Performance monitoring starts for Raw material suppliers Supplier development procedure updated |
| Evidence of implementation file | Supplier development procedure. pdf Performance monitoring for Raw material suppliers xx. pdf | |
| S7 | Action taken to verify the effective implementation of corrective actions | Verify by Internal Audit Check sheet Cum Observation Report and found OK |
| Verify effective implementation of corrective action files | Internal Audit Observation Report pdf |
IATF NC closure examples 2:
Statement of nonconformity
The Process of quality assurance was not found fully effective.
Requirement as per IATF 16949:
Measurement System Analysis (7.1.5.1.1): Statistical studies shall be conducted to analyze the variation present in the results of each type of inspection, measurement, and test equipment system identified in the control plan. The analytical methods and acceptance criteria used shall conform to those in reference manuals on measurement systems analysis. Other analytical methods and acceptance criteria may be used if approved by the customer. Records of customer acceptance of alternative methods shall be retained along with results from alternative measurement systems analysis (see Section 9.1.1.1)
Objective evidence
MSA was not evident for the visual inspection at final inspection stage for the part no Nut Weld for the visual defects-like as dent…
Justification for classification
isolated gap, the concerned person found aware, that Poison testing was done as per customer requirement, no impact on the product & customer, hence minor
| Step No | Step Description | Closure Evidence |
|---|---|---|
| S1 | Correction (Containment) action, including timing and responsible person | Attribute MSA for visual defects for Part xx by Mr……… on Date….. |
| S2 | Evidence of implementation | Attribute MSA for visual defects for Part xx prepared |
| Evidence of implementation file | Attribute MSA for Visual defects for Part xx pdf | |
| S3 | Root Cause Analysis | Why 01: MSA not done for visual inspection for the visual defect. Why 02: MSA about visual defects not defined. Why 03: MSA Plan about visual inspection for visual defects not made. Why 04: In MSA procedure, not defined about MSA Plan for visual defects. |
| Does the root cause impact other similar processes or products? | Yes | |
| Please describe how the root cause impacts other processes. | The Capability of an inspector for visual defects for other parts can not be verified. so that it is required for a similar process or product. | |
| Root cause analysis files | Why why analysis.pdf | |
| S4 | Root cause result | The MSA procedure is not defined about an MSA Plan for visual defects. |
| Root cause result files | Why why analysis.pdf | |
| S5 | Systemic corrective actions, including timing and responsible person | MSA Procedure to be updated by Mr………. on Date………… MSA Attributes plan for Visual Defects to be prepared by Mr………… on Date………… |
| Corrective action files | Corrective action file. pdf | |
| S6 | Evidence of implementation | MSA Procedure updated MSA Attributes Plan for Visual Defects prepared |
| Evidence of implementation files | MSA Procedure.pdf Attribute MSA Plan for visual defects.pdf | |
| S7 | Action taken to verify the effective implementation of corrective actions | Verify the corrective action by Internal Audit Check sheet Cum Observation Report and found OK |
| Verify effective implementation of corrective action files | Internal Audit observation report.pdf |
IATF Closure Time:
In case of minor nonconformity, then IATF NC Closure time is within a maximum of 60 calendar days from the closing meeting of the site audit. If a major nonconformity, then the certification body shall conduct an onsite special audit for the verification of the corrective action. Time to complete the special audit within a maximum of 90 calendar days from the closing meeting of the site audit. In any doubt about closing the Non-Conformity, please feel free to message me.
Read about IATF Rules 6th Edition Q&A Session

I highly recommend this post for effectively managing and closing IATF audit non-conformities. Their structured approach and comprehensive tools streamline the correction and root cause analysis processes.
VERY WELL EXPLAINED ,FIND VERY HELPFUL TO ME ,
Good V I should certainly pronounce, impressed with your web site.
I think other website proprietors should take this website as an model, very clean and excellent user genial style and design, let alone the content. You’re an expert in this topic!