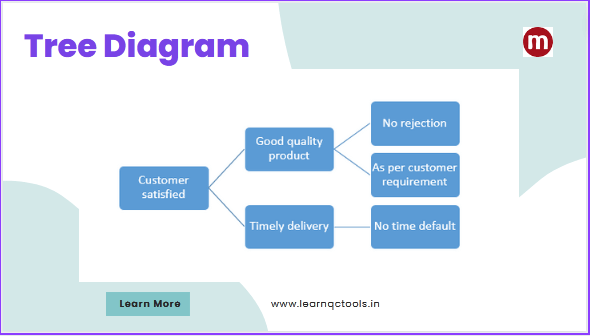
Tool, Fixture & Gauge Maintenance Procedure in Manufacturing
Tools, fixture & gauges are critical assets in any manufacturing organization. Their condition directly affects product quality, dimensional accuracy, productivity, and customer satisfaction. Poor maintenance can lead to defects, rework, downtime, safety risks, and audit non-conformities. This article provides a complete, practical, and ISO/IATF-aligned procedure for tool, fixture, and gauge maintenance, including preventive maintenance planning, … Read more